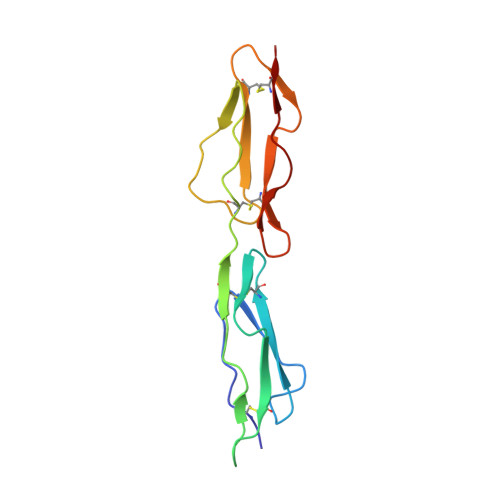
Mapping Cd55 Function. The Structure of Two Pathogen-Binding Domains at 1.7 A
Williams, P., Chaudhry, Y., Goodfellow, I.G., Billington, J., Powell, R., Spiller, O.B., Evans, D.J., Lea, S.M.(2003) J Biol Chem 278: 10691
- PubMed: 12499389
- DOI: https://doi.org/10.1074/jbc.M212561200
- Primary Citation of Related Structures:
1H03, 1H04, 1H2P, 1H2Q, 1UOT - PubMed Abstract:
Decay-accelerating factor (CD55), a regulator of the alternative and classical pathways of complement activation, is expressed on all serum-exposed cells. It is used by pathogens, including many enteroviruses and uropathogenic Escherichia coli, as a receptor prior to infection. We describe the x-ray structure of a pathogen-binding fragment of human CD55 at 1.7 A resolution containing two of the three domains required for regulation of human complement. We have used mutagenesis to map biological functions onto the molecule; decay-accelerating activity maps to a single face of the molecule, whereas bacterial and viral pathogens recognize a variety of different sites on CD55.
Organizational Affiliation:
Laboratory of Molecular Biophysics, Department of Biochemistry, University of Oxford, United Kingdom.