Functional and structural characterization of Streptococcus pneumoniae pyruvate kinase involved in fosfomycin resistance.
Taguchi, A., Nakashima, R., Nishino, K.(2023) J Biol Chem 299: 104892-104892
- PubMed: 37286036
- DOI: https://doi.org/10.1016/j.jbc.2023.104892
- Primary Citation of Related Structures:
8IAS, 8IAT, 8IAU, 8IAV, 8IAW, 8IAX - PubMed Abstract:
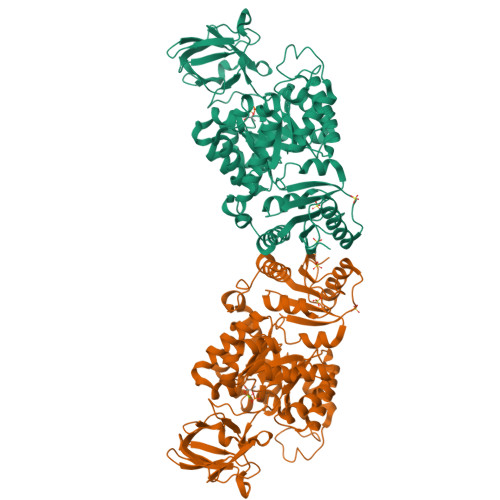
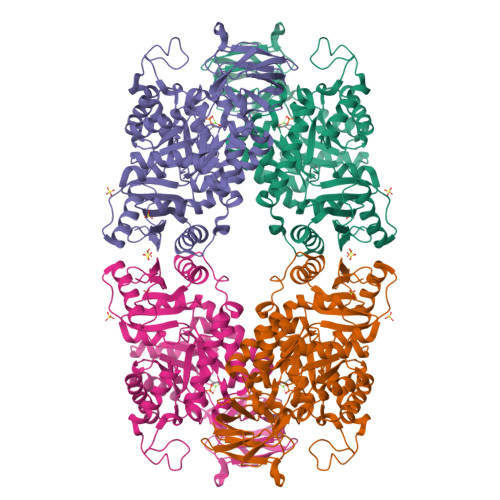
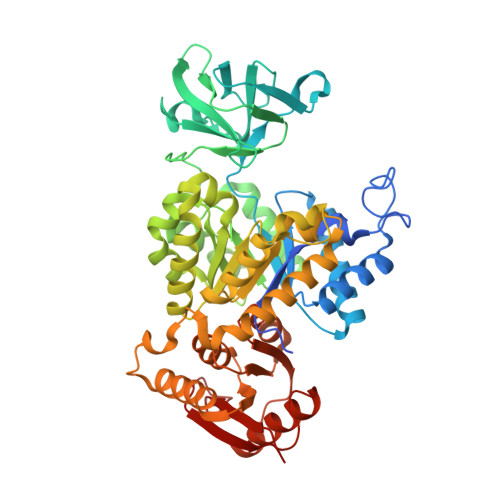
Glycolysis is the primary metabolic pathway in the strictly fermentative Streptococcus pneumoniae, which is a major human pathogen associated with antibiotic resistance. Pyruvate kinase (PYK) is the last enzyme in this pathway that catalyzes the production of pyruvate from phosphoenolpyruvate (PEP) and plays a crucial role in controlling carbon flux; however, while S. pneumoniae PYK (SpPYK) is indispensable for growth, surprisingly little is known about its functional properties. Here, we report that compromising mutations in SpPYK confers resistance to the antibiotic fosfomycin, which inhibits the peptidoglycan synthesis enzyme MurA, implying a direct link between PYK and cell wall biogenesis. The crystal structures of SpPYK in the apo and ligand-bound states reveal key interactions that contribute to its conformational change as well as residues responsible for the recognition of PEP and the allosteric activator fructose 1,6-bisphosphate (FBP). Strikingly, FBP binding was observed at a location distinct from previously reported PYK effector binding sites. Furthermore, we show that SpPYK could be engineered to become more responsive to glucose 6-phosphate instead of FBP by sequence and structure-guided mutagenesis of the effector binding site. Together, our work sheds light on the regulatory mechanism of SpPYK and lays the groundwork for antibiotic development that targets this essential enzyme.
Organizational Affiliation:
SANKEN (The Institute of Scientific and Industrial Research), Osaka University, Ibaraki, Osaka, Japan; Graduate School of Pharmaceutical Sciences, Osaka University, Suita, Osaka, Japan. Electronic address: taguchi@sanken.osaka-u.ac.jp.