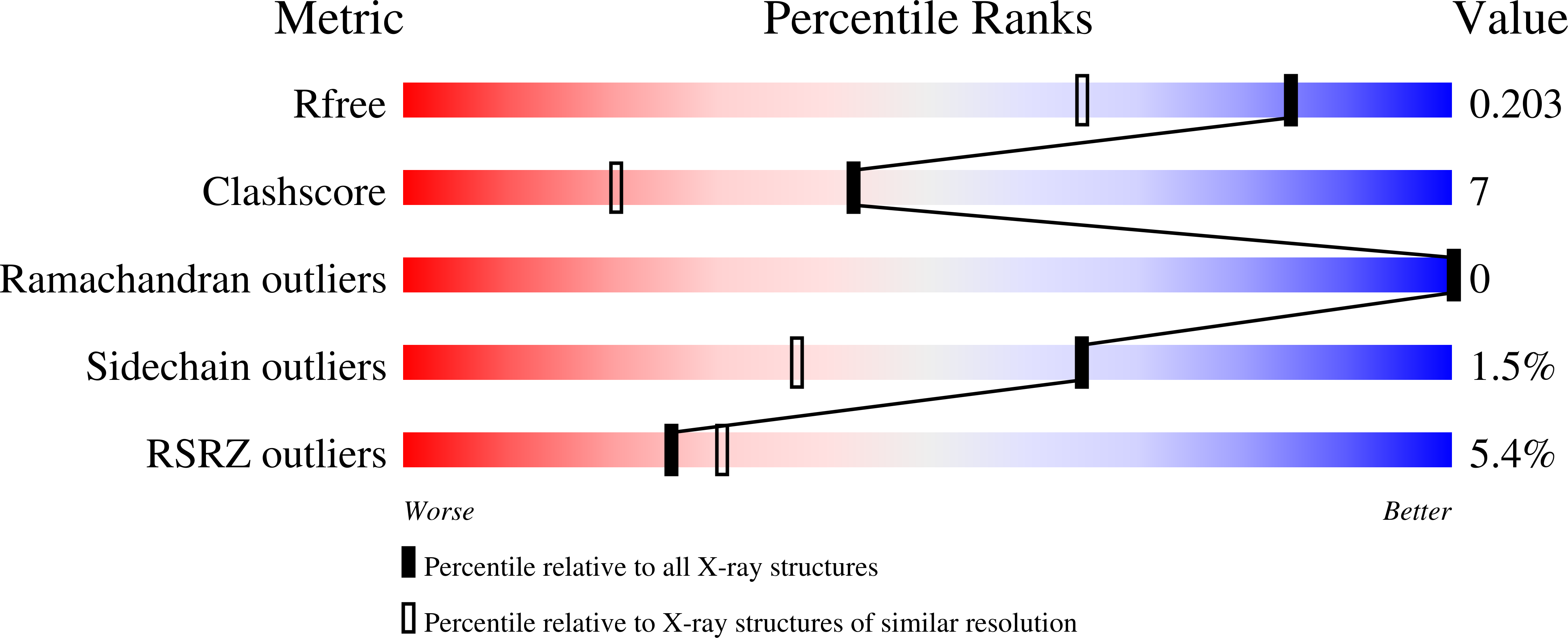
Comparative X-ray analysis of the un-liganded fosfomycin-target murA.
Eschenburg, S., Schonbrunn, E.(2000) Proteins 40: 290-298
- PubMed: 10842342
- DOI: https://doi.org/10.1002/(sici)1097-0134(20000801)40:2<290::aid-prot90>3.0.co;2-0
- Primary Citation of Related Structures:
1EJC, 1EJD - PubMed Abstract:
MurA, an essential enzyme for the synthesis of the bacterial cell wall, follows an induced-fit mechanism. Upon substrate binding, the active site forms in the interdomain cleft, involving movements of the two domains of the protein and a reorientation of the loop Pro112-Pro121. We compare two structures of un-liganded MurA from Enterobacter cloacae: a new orthorhombic form, solved to 1.80 A resolution, and a monoclinic form, redetermined to 1.55 A resolution. In the monoclinic form, the loop Pro112-Pro121 stretches into solvent, while in the new form it adopts a winded conformation, thereby reducing solvent accessibility of the critical residue Cys115. In the interdomain cleft a network of 27 common water molecules has been identified, which partially shields negative charges in the cleft and stabilizes the orientation of catalytically crucial residues. This could support substrate binding and ease domain movements. Near the hinge region an isoaspartyl residue has been recognized, which is the product of post-translational modification of the genetically encoded Asn67-Gly68. The homogeneous population with L-isoaspartate in both structures suggests that the modification in Enterobacter cloacae MurA is not a mere aging defect but rather the result of a specific in vivo process.
Organizational Affiliation:
Max-Planck-Institute for Medical Research, Department of Biophysics, Heidelberg, Germany. [email protected]